Abstract
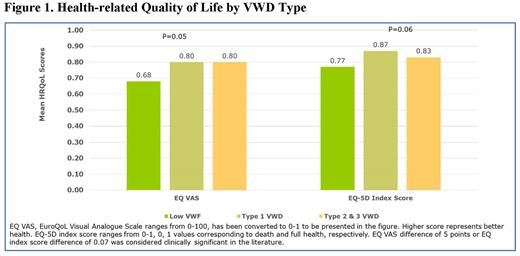
Background: Bleeding disorders substantially impact affected persons' health-related quality of life (HRQoL). However, research exploring factors associated with HRQoL in individuals with Von Willebrand disease (VWD) is limited. Objective: We investigated clinical characteristics associated with HRQoL in persons with VWD who obtain care at seven geographically representative US Hemophilia Treatment Centers. Methods: We enrolled and collected data on individuals age ≥12 with low VWF (VWF:Ag/RCo: 30-50%), VWD Type 1 (VWF:Ag/RCo: ≤30%), Types 2 and 3 between September 2018 - June 2021. Participants completed a survey to collect the following data: sociodemographic and clinical characteristics, healthcare utilization, bleeding episodes during the previous six months, pain and joint health, and HRQoL measured by EQ-5D-3L. Clinical information regarding VWD type and treatment was abstracted from patient charts. Association of participants' sociodemographic and clinical characteristics with HRQoL was assessed using Student T-tests or one-way ANOVA. To compare the HRQoL scores difference between groups, we defined the clinically meaningful difference as minimum 5 points difference for visual analogue scale (VAS), and 0.07 points difference for EQ index score. Results: The sample included 98 participants. Mean age was 31.8 (SD=18.6) years, 67.3% were adults ≥18 years, 79.6% were female, 67.3% had Type 1/low VWF, and 3.1% had Type 3 VWD. Mean EQ VAS was 77.7 (SD=19.5), which is a 5.8 point lower than U.S. population norm of age 25-31 (83.5). Mean EQ index score was 0.84 (SD=0.16), which is a 0.07 point lower than the U.S. population norm of age 25-34 (0.912). Persons with low VWF had significantly lower mean VAS (68.1 vs. 80.5 for type 1, 79.9 for type 2 or type 3, p=0.05) and mean EQ index score (0.77 vs 0.87 for type 1, 0.83 for type 2 or 3 VWD, p=0.06) compared to those with Type 1or Types 2 and 3 VWD. Adults 18 years or older had a 6.9 point lower mean EQ VAS compared to participants age 12-17 years old (75.5 vs 82.4, p=0.10). Mean VAS was an 8.4 point lower among unemployed persons than the employed (71.4 vs 79.7, p=0.14). Mean VAS was an 8.1 point lower among individuals reporting recent bleeding events (72.8 vs. 80.9, p=0.047) and mean EQ index score was a 0.07 point lower (0.79 vs. 0.86, p=0.05) than among those without bleeding episodes. Mean EQ index score was significantly lower in persons with anemia than those without (0.80 vs 0.87, p=0.03). Persons reporting joint problems had both a significantly lower mean VAS (73.2 vs. 81.9, p=0.03) and a lower mean index score (0.15 difference, 0.75 vs. 0.90, p<0.0001). Persons with VWD reporting a medical procedure within the last 6 months had a 9.7 point lower mean VAS (70.6 vs. 80.3, p=0.07), and a 0.06 point lower mean EQ index score (0.79 vs 0.85, p=0.13) than those with no medical procedures. Individuals reporting hospitalization in the prior 6 months had a 5.6 point lower mean VAS score (72.7 vs 78.3, p=0.57), and a 0.11 point lower mean EQ index score (0.74 vs 0.85, p=0.06) than those without hospitalization. Females reported a 0.08 point lower EQ index score as compared to male (0.82 vs 0.90, p=0.02). For women with VWD, those reporting heavy menstrual bleeding (HMB) had a 7.7 point lower mean VAS (75.5 vs. 83.2, p =0.23), and a 0.08 point lower mean EQ index score (0.80 vs 0.88, p=0.04) than those without HMB. Conclusions: Our study confirms VWD impacts HRQoL, and multiple factors as observed in bleeding disorders in general (older age, recent bleeds, joint problems, anemia, female gender, and HMB in women) are negatively associated with HRQoL in persons with VWD. In addition, a diagnosis of low VWF, recent medical procedure and hospitalization also impact HRQoL. Further study is in order in better understanding these risks in the VWD patient in hopes of developing interventions that ultimately improve HRQoL.
Disclosures
Carpenter:genentech: Honoraria. Wu:Baxalta US Inc., Bannockburn, IL (a Takeda Company), CSL Behring L.L.C., and Octapharma USA, Inc.: Research Funding. Roberts:Takeda, Genentech; Speakers Bureau: Novo Nordisk, Sanofi, Takeda: Research Funding; Novo Nordisk, Octapharma, Pfizer, Sanofi, Takeda, Genentech, Novartis: Consultancy. Kulkarni:Sanofi: Consultancy; Roche: Consultancy. Sidonio, Jr.:Biomarin: Honoraria; Genentech: Honoraria, Research Funding; UniQure: Honoraria; Bayer: Honoraria; Novo Nordisk: Honoraria; Octapharma: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Pfizer: Honoraria; Spark: Honoraria; Guardian Therapeutics: Honoraria. Konkle:CSL Behring: Honoraria; Spark: Honoraria; BioMarin: Honoraria; Uniqure: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Sigilon: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Baxalta: Research Funding. Curtis:Baxalta US Inc., Bannockburn, IL (a Takeda Company), CSL Behring L.L.C., and Octapharma USA, Inc.: Consultancy; Bayer, and Novo Nordisk.: Consultancy. Nichol:Pfizer, Genentech Inc., Baxalta US Inc., Bannockburn, IL (a Takeda Company), Octapharma, CSL Behring, and Global Blood Therapeutics.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.